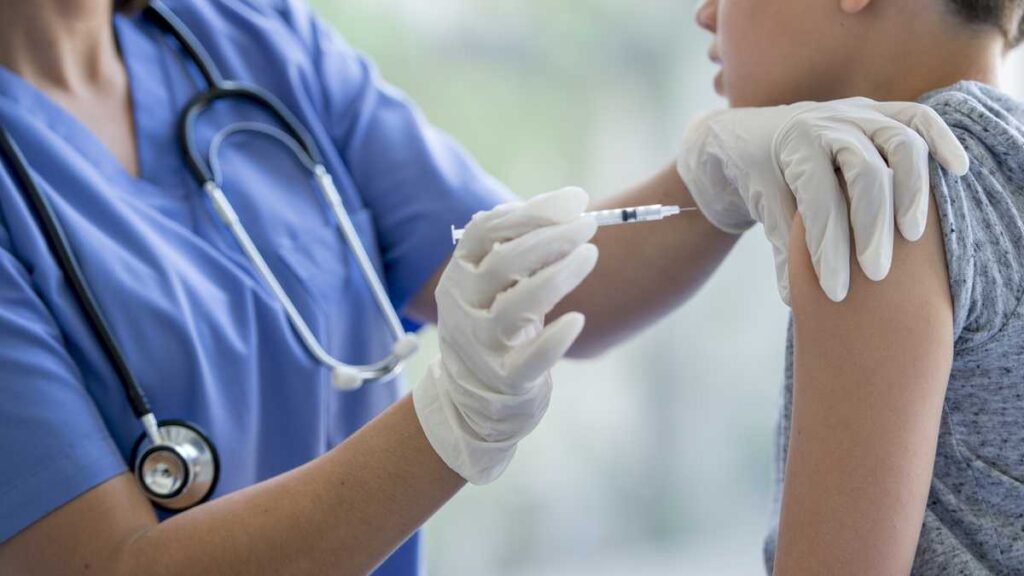
FDA TO APPROVE COVID-19 VACCINE FOR CHILDREN AGES 5-11 BY THE END OF OCTOBER.
Newsroom El Comercio de Colorado
Haga click aquí para leer la versión en español
Pfizer formally applied to the FDA for authorization for the emergency use of its COVID-19 vaccine for children ages 5 to 11. The Dose for minors will contain 10 micrograms, in contrast to the 30 micrograms that adults receive. The drugmaker has already announced a robust antibody response in children. Experts estimate that the FDA will issue its decision before the end of October and the vaccine will be applied in early December.






otras noticias
COVID-19 Affected Individuals Live with its Effects
The gorillas of AMLO
Celebrating the Board of Directors (2024-2025) of the COHCC.